Veritalk: Monsters Episode 2 - Parasites
Some monsters live inside us. PhD candidate in Biological Sciences in Public Health Maddy Mcfarland studies Trypanosoma cruzi, a parasite that transforms its shape to sneak inside our cells and makes us sick. The scariest part: Our cells can’t signal that they’re infected until it’s too late.

What if I told you that there's a monster that can change its shape to better invade our bodies? You'd probably tell me that it's just science fiction. But Maddy McFarland, a PhD student in Biological Sciences in Public Health, studies a parasite that is so good at hiding in our bodies that it can float in our cells, undetected, for decades at a time. This week on Veritalk: Parasites.
Subscribe to Veritalk
Full Transcript

Voices: I mean, why?
Anna Fisher-Pinkert: From the Harvard Graduate School of Arts and Sciences, you’re listening to Veritalk. Your window into the minds of PhDs at Harvard University.
Voices: I was curious. . . I've always wondered. . . why?
AFP: Last week, we talked with Greg Darwin about creatures that live on the edge of humanity.
Greg Darwin: You go to the shore you go out to sea and you're kind of stepping outside of the bounds of your familiar village, you go into the woods you might not come back.
AFP: Greg talked to us about mermaids and selkies. And he said these legendary sea monsters are just long-lost cousins of our modern-day urban legends. They’re intrusions of the supernatural into our every day lives.
GD: Regardless of our advances in technology, and our advances in general quality of life that most of the world is enjoying, we're still basically humans and we're still worried about our place in the world.
AFP: But, some of the stuff that scares us isn’t fictional at all. There are some very real, very nasty things that can invade our bodies. They’re what we call parasites. And though they are much smaller than your average sea monster - they’re no less terrifying.
Madalyn McFarland: So you know, worms for example: You do see some interesting features that are a little more scary. You know the the the part of their mouth that is used to grab onto the intestine, for example, looks quite a bit like something that you'd see in a horror movie. However, instead of being a foot like diameter it's you know two or three millimeters. [laughs] So, it's much, much smaller.
AFP: That’s Maddy McFarland.
MM: I am a graduate student in my second year. I work in the laboratory of Barbara Burleigh, who studies Chagas disease caused by Trypanosoma cruzi. And I am in a program called BPH, biological sciences in public health.
AFP: Madalyn’s program is a collaboration between the Harvard Graduate School of Arts and Sciences and the Harvard TH Chan School of Public Health. The creature she studies isn’t an intestinal worm. It’s much, much smaller. But, it also has the potential to be absolutely deadly: it’s a parasite called Trypanosoma cruzi.
MM: Trypanosoma cruzi. You might have heard the word tripanasoma in association with Trypanosoma brucei, that parasite is a very closely related parasite which causes African sleeping sickness.
AFP: Tripanosoma cruzi causes something called Chagas disease. What’s scary about Chagas disease is that you might not even know that you’ve been infected - until it’s too late.
MM: Typically acute infection either has no symptoms or just very general symptoms much like a cold or the flu would.
AFP: Some people can go their entire lives after infection without any symptoms.
MM: But in a small percentage of cases cases, about 30% in their lifetime, will develop a form of cardiac Chagas disease which will cause disease throughout the entire heart. Or, they'll develop some sort of intestinal dysbiosis. So they'll have mega colon or mega esophagus caused by the parasite disrupting the nervous system in the digestive system.
AFP: If you manage to catch Chagas disease during the acute phase, treatment is 60-90% effective.
MM: Now, keeping in mind most people don’t know that they’re acutely infected.
AFP: Once the disease reaches the chronic phase, treatment is barely effective.
MM: During the chronic phase, it's all over the place - unlikely to be super efficacious any more so than symptomatic treatment would be. So, knowing that once you've had it for, you know, say eight weeks, there is very little treatment for it, or very little ability to even be able to address any of your symptoms without, you know, drastic care - that's a little bit scary. And so the development of new drugs, particularly for chronic chagas diseases. is a major issue.
AFP: So Maddy is on a mission to hunt down a deadly organism that can live inside our bodies, undetected, for decades at a time. And, like Van Helsing or Buffy, or any good monster hunter, she has to find its weaknesses to take it down. Just a warning to our listeners, the next couple of minutes are going to be a little bit gross. I promise that there is some really interesting science on the other side. Just put away that sandwich. I asked Maddy to explain how Trypanosoma cruzi gets into our bodies.
MM: There's this bug called the kissing bug which is about the size of a penny or can grow to be about the size of about an inch long. They live throughout South America and Central America and up to about a line which crosses the United States around New Jersey. There are hundreds of different species of this bug but only about 12 of them can carry Chagas disease.

AFP: Scientists originally discovered Trypanosoma cruzi, the parasite that causes Chagas disease, inside the kissing bug. The parasite doesn’t do much damage while it’s in there - the kissing bug is basically a taxi service, delivering it to its target hosts: us.
MM: And so these bugs whether they're infected or not try to live in different areas that humans might be. So they'll invade housing units that don't have sealed cracks and they'll live in the cracks of housing units. And then at night, they'll come out to feed because they're primarily blood feeding bugs, and they'll bite mammals of any variety, including humans. Typically what happens to a human is they'll get it on the face area. And so they'll bite somewhere near the eyeball or the mouth. Because the bug is eating, it will defecate and the person will inherently go to scratch the bite or move the bite or something like that, and they'll push the feces into the wound or into the eye. And that will actually cause the infection.
AFP: Like I said, pretty gross. But here’s the science I promised: Trypanosoma cruzi has something in common with Greg Darwin’s selkies. Like a selkie putting on her cloak to return to the sea, the parasites change their gene expression to be able to navigate different environments.
MM: I'd say that parasites in general have a lot more genetic space to move around in because their genomes are larger than a bacteria, for example. So a parasite like Trypanosoma cruzi actually has many, many more genes and many genes families. So there are genes that are very closely related but they can switch which ones they're expressing in order to change things like their surface and things like that. So they may be perfectly genetically identical, these parasites. You have, you know, two sibling parasites - and they will be able to look completely different.
AFP: Ok, so I really wanted Trypanosoma cruzi to like a microscopic Xenomorph chasing down microscopic Sigourney Weaver, but Maddy says it’s all a little subtler than that.
MM: A parasite's a parasite no matter where it's at. So it's going to be genetically identical. But the thing that is capable of changing is just it's it's outside appearance. So these parasites they remodel their outside surfaces very quickly throughout their life phases. So there are about four main stages that are important.
AFP: The first stage is known as the “epimastigote phase.”
MM: So the epimastigote phase is just the name of the parasite life stage which lives in the bug. It's a replicative stage so they will divide.
AFP: Trypanosoma cruzi reproduces asexually - so dividing is the only way it can make more of itself.
MM: Then they will eventually convert once they move down the midgut into the hindgut where they would get into the feces. They'll convert to what's called a metacyclic trypomastigote. This just means it's capable of infecting infecting humans, so they look very different.
AFP: During the metacyclic trypomastigote phase, the parasite remodels its long flagella, which looks a bit like a tail. It uses that tail to swim through the human bloodstream, looking for a host cell.
MM: They come up to the host cell invade as a trypomastigote, the motile, non-dividing form - so these do not divide. They'll round up like a little snake would, like kind of in a circle. And then they'll slowly convert over the next 18 hours into an amastigote. It has, instead of a very long flagella, which they used to run around, just a very short little stubby flagella, So that is kind of the amastigote -- doesn't have a flagella. And at that point they'll wait for just a little bit until they're fully converted, and they will again begin replicating.
AFP: Now, scientists yet know how this happens - but after filling the host cell with duplicates of itself, Trypanosoma cruzi receives some sort of signal, and starts to change yet again.
MM: Then they'll reconvert into trypomastigotes. And then again become motile - so they'll move, they'll move, they'll move. And we don't know exactly what triggers this, but then they'll burst the host cell and all swim out and invade other cells.
AFP: That’s one parasite with four different transformations. And each transformation comes with new superpowers that allow the parasite to thrive in hostile conditions.
MM: So first they're going to be a bug, which is going to be a fairly hostile environment. They're not going to be protected from anything that's going on in that bug. So any bacteria that are in their microbiome can cause problems for them. In the midgut it's got to be able to survive and be able to get enough nutrients to replicate many, many times. And it's not a particularly rich environment unlike the human host cell.
AFP: During the typomastigote phase, the one with the special long flagella, it’s more important to be a good swimmer than a good eater.
MM: So its main task at this point is to get into the feces, and then be put into some sort of mammalian host. So at that point, the only thing it needs to do is remain motile and not waste energy, essentially. So it just moves around as fast as it can for as long as it can and then it will eventually die if it's not put into a human host. But in the human host it has a very unique environment.
AFP: Most parasites keep a protective membrane around themselves when they are inside the host cell. But Trypanosoma cruzi floats around without any membrane at all. It's inside the cytosal of the host cell.
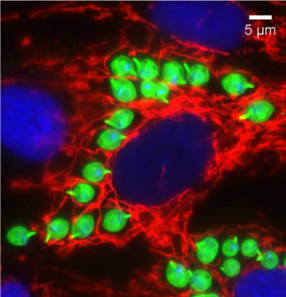
MM: So the cytosal is basically just the liquid gelatinous material inside of our cells that contains all that nutrients and proteins. But also a lot of our defenses against the against parasites and different invaders are also located in the cytosal. So it's got to be able to kind of balance between getting the nutrients, and defending itself, and also being able to replicate. So it's a very different environment than it was facing even in the insect form.
AFP: The human body does have a pretty robust defense system - but once Trypanosoma cruzi is inside the host cell, immune cells like T cells and B cells can’t get to them. Cells do have some of their own defenses.
MM: One thing that the cell could do is it could break up that parasite or cause some sort of degradation and then tell the rest of the immune system that it's infected by putting something out on its MHC presenting molecules.
AFP: MHC molecules are proteins that the cell can use to talk to some immune system cells.
MM: But, it doesn't do that. It has no way to tell the rest of the body, "Hey I'm an infected cell," that we know of. So essentially the parasite is able to disguise itself well enough, or reprogram the cell well enough, that the cell doesn't tell anybody else that it's infected.
AFP: It’s like a microscopic version of invasion of the body snatchers. The body doesn’t recognize infected cells until the invaders are everywhere. And even when the immune system does fight them off - a few infected cells can remain undetected, just waiting for the right moment to strike.
[Laughter]
AFP: Excellent, I'm going to switch this. . . test, test.
AFP: So, obviously, I had to face down these monsters.
MM: Yeah, so we are at the School of Public Health. . .
AFP: Maddy leads me to a microscope in her lab where I can see Trypanosoma cruzi in the flesh - well, ok, in a prepared sample.
MM: So, these are epimastigotes, these are just wild type regular parasites. You can see them, they're kind of long. They move around and they follow their flagellas. Can you see those?
AFP: Oh, oh yeah!
MM: So they're pretty quick little guys. They swim around. They look a little bit like tadpoles, but they swim towards their flagella.
AFP: Oh, so like a backwards tadpole.
MM: Yeah, exactly
AFP: The little tail is in the front.
MM: Yeah.
AFP: Sitting there under a microscope, Trypanosoma cruzi doesn't look all that terrifying. But I also know what it's capable of. I asked Maddy if, after all this time studying parasites, she thought they were more or less scary.
MM: Kind of both. I'd say I've learned a lot more about what could happen, but I've also learned a lot about the statistics of it happening. So the odds of you actually having this disease plus having all these complications plus all these things is pretty low, right? Not necessarily just Trypanosoma cruzi, but different parasites. However I have also learned about the efficacy of the drugs we use to treat these parasites, and it's not great. [Laughs] We wish it was better. And that's a lot of our goal in the end is to be able to develop new drugs.
AFP: It does seem like it's some of these horror things that we think about some of the things we're afraid of, it seems like these are big exaggerations of things that people have known were scary or dangerous for a long time.
MM: Right. Right it makes it scarier in a more immediate sense when it comes bursting out of someone.
AFP: Yeah, yeah!
MM: But that's I'd say that's pretty rare in the parasite world. You might have some things that you know you pop out of your skin somewhere or something that would be an abnormal parasite that's not your typical parasite. You can think of many, many things that are parasites, actually. So hookworm, even malaria is a parasite. Things that are actually very scary on a global level. But these movies, they take the scary parts that are more like the absolute tiniest part of truth, and exaggerate them a thousand times.
AFP: So where does science fiction end, and science begin?
Shane Campbell-Staton: I have no idea how long it would take me to eat 8,000 lbs of food.
AFP: Next time on Veritalk, we’re talking with Shane Campbell-Staton of the podcast the Biology of Superheroes. And we’re going from small, to big. Very, very big.
SCS: Every single day, that's what it has to do to stay alive and keep swimming.
AFP: That’s next time on Veritalk. Veritalk is produced by me, Anna Fisher Pinkert. Our sound designer is Ian Coss. Our logo is by Emily Crowell. Our executive producer is Ann Hall. Special thanks this week to Maddy McFarland, Julia King, Barbara Burleigh, and the National Institutes of Health, which funds the research of the Burleigh lab. Don’t forget to subscribe to Veritalk wherever you get your podcasts - and rate and review us while you’re there! It really makes a huge difference. If you have comments or questions for us - or if you want to see pictures of Trypanosoma cruzi, visit gsas.harvard.edu or send us an email at veritalk@fas.harvard.edu. Thanks for listening.
Logo by Emily Crowell
Get the Latest Updates
Join Our Newsletter
Subscribe to Colloquy Podcast
Simplecast