A Better Way to Treat Obesity
New research targets genetic causes of the disease
Obesity is killing us—literally. A 2022 study in the British journal The Lancet found that being overweight contributed to more than 1,300 excess deaths per day in the United States in 2016. The disease is also bankrupting us. One study put the aggregate medical costs of obesity in the US at $260 billion per year.

It’s no surprise, then, that glucagon-like peptide-1 (GLP-1) agonist drugs like Ozempic and Wegovy—which can induce weight loss of 15 to 20 percent—have exploded in popularity. According to the independent nonprofit FAIR Health, use of these medications to treat overweight or obesity in the US increased nearly 600 percent from 2019 to 2024. But this class of drugs was originally developed to treat diabetes, not obesity. In fact, Ozempic is still not approved by the US Food and Drug Administration for weight loss, and those who take the drug to lose weight often experience a raft of debilitating side effects.
Muhammad Ahmad is looking for a better way to treat obesity. Using the functional genomics (gene-editing) technology CRISPR-Cas9, the PhD student at Harvard’s Kenneth C. Griffin Graduate School of Arts and Sciences (Harvard Griffin GSAS) has pinpointed the genes that regulate the retention and burning of fat in fruit flies and mammalian cells. With further testing, Ahmad’s work could lead to safer, more effective therapies for one of the greatest challenges to health in the developed world.
Controlled Burn
Ahmad focuses on cell signaling. Specifically, he and his colleagues study the hormone glucagon, which increases when organisms starve.
“Glucagon’s targets are fat cells and liver cells, the two metabolically active sites in your body that store excessive fat,” he explains. “So, when you’re starving, this hormone tells these cells to release stored fat. That's how weight loss happens.”
The signal to release stored fat actually comes from the glucagon receptor, a class of G-protein-coupled receptors (GPCRs), expressed on liver and fat cells, to which glucagon binds, a bit like a key in a lock. Because GPCRs are the most common receptors in the body, many FDA-approved medications target them to induce weight loss. “But the medications may not be able to distinguish between GPCR agonists, which induce signaling, and antagonists, which effectively block it,” Ahmad says. “That leads to side effects.”
Ahmad’s team wanted to identify new regulators of glucagon hormone receptors—ones that specifically tell cells to break down stored fat. The idea was that, if the researchers could more precisely identify the regulators, they could enhance cell signaling, increasing fat burning specifically in adipose tissue and hepatic tissue, eventually causing weight loss.
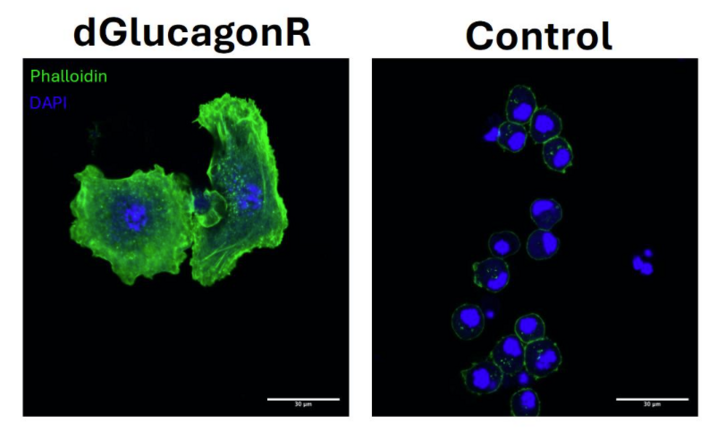
Using high-throughput, high-precision, CRISPR Cas-9 gene-editing technology, Ahmad’s team one-by-one knocked out thousands of genes present in the DNA of fruit flies, then observed how the flies responded to the changes. “If a deletion resulted in increased storage of fat, then we would know that, if the gene was normally present, it would improve fat breakdown and, eventually, weight loss,” he explains.
By the time Ahmad’s team finished its screen of the glucagon hormone receptor, they’d identified a lot of well-known factors in fat burning, evidence that the process was accurate. But they also identified with high confidence new factors important for the stability of the glucagon hormone. “We now have a very clear picture of how controlling just one lipid factor on the receptor improves its stability,” he says. “We can target these particular factors to enhance the signaling. We have seen the impact on flies. If we remove these factors, the fly becomes super obese very quickly.”
We now have a very clear picture of how controlling just one lipid factor on the receptor improves its stability. We can target these particular factors to enhance the signaling. We have seen the impact on flies. If we remove these factors, the fly becomes super obese very quickly.
—Muhammad Ahmad
Because the metabolic processes he studies are also present in mammals, Ahmad says his team’s findings should translate to mammalian systems. “We find very similar effects in individual mouse cells. The next step is real-time tests in the animals themselves.”
Harvard Medical School Professor Norbert Perrimon, Ahmad’s faculty advisor, calls the PhD candidate’s obesity research “cutting edge.” “Muhammad has uncovered novel signaling components and regulators of glucagon signaling in fruit fly cells,” he says. “Through this work, he identified several genes involved in signaling and presented strong evidence that palmitoylation (where a fat molecule is added to a protein) plays a critical role in signal transmission. These findings are significant and open up promising new avenues for targeting glucagon signaling in the context of obesity and diabetes.”
How High-Protein Diets May Work
Ahmad's research also led to an unexpected discovery. In a paper published in Nature Communications in January 2025, he and his colleagues revealed that glucagon is also activated by a high-protein diet. The finding could help explain the success of such regimens in weight loss—a topic of debate among researchers for years.
“It has been a longstanding belief in endocrinology that glucagon increases only under nutrient starvation, but we are showing that’s not true,” he says. “The response is triggered by specific amino acids present in the diet as well. It’s far more nuanced than it was thought to be.”
Almost simultaneously, researchers in the lab of Robert Holmgren, PhD ’80, a professor in Northwestern University’s Department of Molecular Biosciences, were making much the same discovery—an indicator of the robustness of Ahmad’s findings.
“Muhammad Ahmad and the Perrimon lab have a longstanding interest in organismal-level regulation of metabolism in Drosophila,” says Holmgren. “My group studies Hedgehog signaling, and we were following up on some work by the late Dr. Suzanne Eaton’s lab on the role of Hedgehog signaling in lipid metabolism. That we both also came across the surprising role of calcium waves—which spike once glucagon has activated its receptor, triggering the Drosophila fat body starvation response—is very satisfying.”
Anxious about Agonists
Ahmad didn’t start out to study obesity. During an undergraduate internship in Pakistan, he got interested in behavior and how it might be influenced by food—or its lack.
“I identified that when you starve animals, which are readily accepted to be vegetarian, they start attacking their own eggs,” he recalls. “I’m talking about insects, mostly. That came out as a first-author paper directly from my undergraduate work. I was doing it for fun.”
The excitement of his college research inspired Ahmad to enroll as a PhD student at Harvard Griffin GSAS. He did so originally intending to study neuroscience, particularly the circuitry of the brain. He rotated through labs conducting studies of cerebrospinal fluid, sleep, and vagus nerve sensation. On his next rotation, however, he decided to take a break from neuroscience and work with a group studying metabolism and inter-organ communication. “That changed everything,” he says. “I was so excited to learn how organs communicate with each other and how that could be finely tested with an eye toward coming up with solutions to many problems [for human health].”
Ahmad quickly began to see that obesity, heart disease, and many other ailments couldn’t be understood or treated by looking at one organ or tissue in isolation. “For example, when fat storage increases in your adipose tissue, it has a direct impact on your blood,” he explains. “LDL cholesterol is affected. And then eventually your heart. There’s also a concept called dyslipidemia, where the imbalance in lipids spreads like a cancer to other organs.”
Just as Ahmad discovered his passion for the study of metabolism and inter-organ communication, GLP-1 agonists like Ozempic were gaining momentum as treatments for obesity. The drugs effectively curb appetite, promote feelings of fullness, and lower post-meal blood sugar spikes. Unfortunately, they may also induce nausea and vomiting, cause acute pancreatitis, and cause users to shed muscle as well as fat. Additionally, not all patients respond equally well to these treatments.
My dream is that a pharmaceutical company picks up the targets we’ve identified . . . and feeds them into a drug development pipeline. Then we can come up with a safe, long-term solution to obesity that doesn’t rely on hormones with harmful side effects.
—Muhammad Ahmad
“These are all indications that we need to approach weight loss from a very different perspective than just injecting a flood of hormone into the body, which can create a lot of off-target effects,” Ahmad says.
Moreover, if a user who is genetically predisposed to obesity discontinues the drug, the excess weight will return. “If your genetics are different, if your body is genetically wired to store a lot of fat, then Ozempic is just a momentary remedy,” he says. “It will work for a time, but the moment you stop taking it, your genetics will kick in again and start making your body store fat.”
There’s no data on the long-term use of GLP-1 agonists, a relatively new class of drugs, so clinicians can’t predict the effects, a particular concern for sufferers from Ahmad’s part of the world. He points out that his home country of Pakistan has the world’s highest incidence of diabetes and that Pakistanis, as well as Indians, are at increased risk of obesity—a consequence of history. “In countries that have experienced a lot of famine in the past, especially in South Asia, bodies rewired their metabolic genetics to store as much nutrient as possible as a survival mechanism. As a result, many people in South Asia are at risk of obesity regardless of their lifestyle or calorie intake.”
Beyond Obesity
Ahmad cautions that research on the genetic origins of obesity is still in the early stages. But he is bullish about the potential of this work, not only for treating obesity but also for other diseases. "Recently, a team of researchers at a nearby hospital contacted us about the genes our group had identified," he says. "They were studying neurodegenerative diseases like Parkinson’s, and they found that these same genetic components that stabilize the same class of proteins as glucagon—GPCRs—might also stabilize certain brain receptors. That could prevent the buildup of toxic proteins in the brain, which is what causes Parkinson’s.”
Ahmad says the collaboration is now moving forward, with experiments planned to test whether the same genes can be protective in neurons as they are in fat cells.
As his dissertation work wraps up, Ahmad is already thinking about where to take the research next—and how to bring it to the people who need it. “My dream is that a pharmaceutical company picks up the targets we’ve identified, which are conserved in humans, and feeds them into a drug development pipeline,” he says. “Then we can come up with a safe, long-term solution to obesity that doesn’t rely on hormones with harmful side effects.”
Ahmad acknowledges that funding for this type of research is always a challenge. He’s grateful for a predoctoral fellowship from the American Heart Association and for support from the Howard Hughes Medical Institute and the National Institutes of Health that have enabled his work. He says that as long as the scientific community keeps working—and keeps listening to the data—the future of obesity research looks promising.
“We’re learning that obesity isn’t just about what you eat or how much you exercise,” he says. “It’s also about what your body is programmed to do. And if we can change that programming, we can change everything.”
Get the Latest Updates
Join Our Newsletter
Subscribe to Colloquy Podcast
Simplecast



