From Bach to Breakthroughs
Musically-inclined PhD student leads effort to develop gene-insertion technology that offers new hope for sufferers of diseases like cystic fibrosis
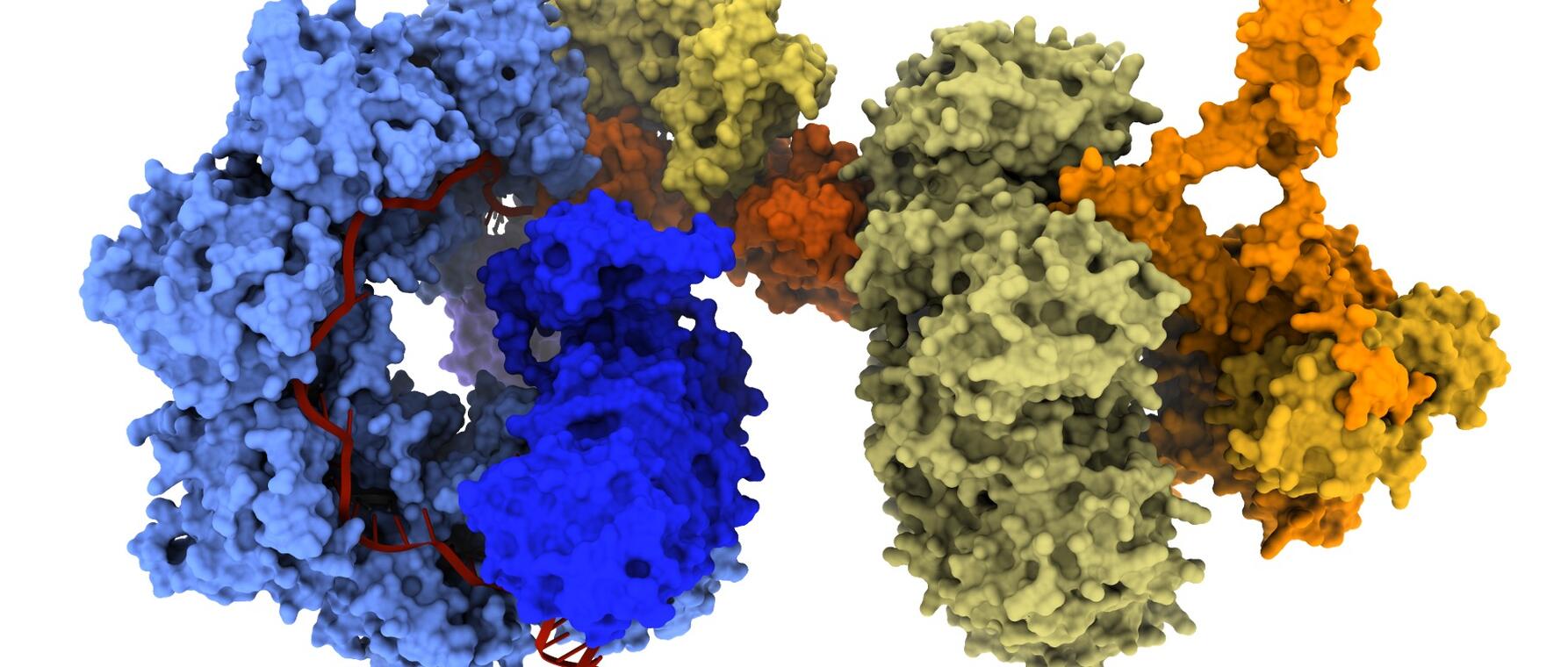

Gene editing promises a future where we can treat devastating diseases by editing and correcting errors in the body’s genetic “blueprint.” One great challenge to this approach, however, is scale. Hundreds of small "misspellings" in a gene can cause a particular genetic disorder, like cystic fibrosis, for example. Editing each genetic misspelling individually would be a tall order, requiring scientists to develop strategies for many specific mutations—and get regulatory approval for each.
But what if we could do more than edit single mutations—what if we could insert an entire healthy gene directly into human DNA, right where it’s needed?
That’s the possibility unlocked by evoCAST, a groundbreaking gene-insertion technology developed by Harvard Griffin GSAS PhD candidate Isaac Witte and colleagues. Working in the lab of David Liu, the Thomas Dudley Cabot Professor of the Natural Sciences, Witte has helped evolve a tool that may bring us closer to efficiently treating diseases like cystic fibrosis and hemophilia at their genetic root—not through the laborious, unscalable process of correcting individual misspellings one by one, but by simply inserting a healthy DNA sequence into the genome.
A Complex Transposition
Witte’s research addresses the genetic causes of disease through a new method of gene editing called evoCAST (evolved CRISPR-associated transposase). Deoxyribonucleic acid (DNA) encodes genes that form the blueprint for how our cells—and thus, our bodies—function. “Misspellings” in that blueprint—mutations—can result in disease.
Witte’s advisor, David Liu, has been a pioneer in using CRISPR (clustered regularly interspaced short palindromic repeats) technologies, namely base editing and prime editing, to develop gene-editing techniques that correct the small sequence changes that give rise to illness. An alternative approach is to install a healthy copy of the gene rather than correcting each mutation. “This is part of the broader notion of treating disorders with gene therapy,” Witte explains. “It’s been around for decades, but the ability to install that sequence within the genome—to put it right where we want it to be—has been difficult.”
In a study published last May in Science, Witte and his coauthors detailed a breakthrough in gene insertion for human cells that uses a bacterial system called CRISPR-associated transposases (CASTs) to insert DNA into the genome.
“There are two sides to this platform,” Witte says. “There’s a DNA search function performed by a CRISPR complex to help locate the target site. Then there’s a module to integrate healthy DNA. That’s the transposase complex.”
Unlike traditional CRISPR-Cas9 systems, which use “molecular scissors” to generate small DNA sequence changes, CAST systems do not require cutting. Instead, CAST “parks” on the target site, recruits the transposase module, and facilitates insertion of the healthy gene.
[Installing a healthy copy of the gene rather than correcting each mutation] is part of the broader notion of treating disorders with gene therapy. It’s been around for decades, but the ability to install that sequence within the genome—to put it right where we want it to be—has been difficult.”
—Isaac Witte
“With evoCAST, we are not directly correcting disease-causing mutations," he explains. "Instead, we install a healthy gene copy that can complement the defective gene. This strategy could serve a much broader patient cohort, as it works agnostic of a patient's specific mutation within the gene."
Accelerating Evolution
Developed in collaboration with the lab of Professor Sam Sternberg at Columbia University, evoCAST was built using phage-assisted continuous evolution (PACE), a platform pioneered by the Liu lab.
In nature, evolution happens slowly, over millions of years, as beneficial mutations are passed down through generations. PACE accelerates this process dramatically. By using bacteriophages and E. coli, researchers can evolve proteins with new functions in just hours.
“CASTs offer the remarkable ability in bacterial cells to perform RNA-programmed integration of gene-sized DNA segments—thousands of base pairs—with high efficiency and accuracy into a specified core in the bacterial genome,” explains Professor Liu. “In mammalian cells, however, the activity of natural CASTs is very low—typically 0.1 percent or less. Isaac and the rest of the team used our PACE system to systematically solve this problem.”
The result? “evoCAST performs useful levels of targeted gene integration in human cell types,” says Liu, “improving their efficiencies by more than 400-fold over the natural starting CAST.”
Columbia’s Sternberg says evoCAST represents a major leap for gene editing. “By harnessing a combination of directed evolution and rational engineering, the technology dramatically expands the scope and precision of CRISPR-associated transposases, enabling targeted gene insertion in ways that were not possible before,” he says. “We are especially excited about the therapeutic potential of evoCAST to correct challenging and heterogeneous loss-of-function genetic diseases.
As lead first author of the study published in Science, Witte was a driving force behind evoCAST, Sternberg says. “He not only conceived and executed many of the key experiments, but also brought together collaborators across labs to move the project forward. His creativity, rigor, and persistence were essential to the success of the work.”
evoCAST performs useful levels of targeted gene integration in human cell types, improving their efficiencies by more than 400-fold over the natural starting CAST.
—David Liu
Witte cautions that evoCAST may be more well-suited for treating certain classes of mutation over others. “Cystic fibrosis, for example, is a disorder caused by the loss of function of a protein. So if we can just provide the cells with the healthy protein, the loss of function can be restored, thereby treating the disease.” Witte explains. “There are other genetic disorders that have a dominant-negative effect, though, where the mutated copy of the gene produces a protein that actively causes dysfunction. That would be more difficult to repair with gene insertion methods like evoCAST.”

Top Performer
Given his upbringing, it seemed more likely that Witte would end up in a concert hall than a laboratory. His mother was a professional oboist; his father studied conducting and French horn. His grandparents on both sides were musicians as well.
Witte took up cello and piano as a child and became an accomplished musician. In high school, a beloved biology teacher sparked a new passion. At the University of California, Berkeley, Witte majored in both biology and music before deciding to pursue a PhD in chemistry and chemical biology at Harvard Griffin GSAS.
Today, he continues to perform with local ensembles, including the Du Bois Orchestra, the Apollo Ensemble, and Harvard’s Graduate Student Orchestra. “In these ensembles, there are people like me, who have other careers but are still passionate about music, along with many music students, teachers, and professionals in the area,” he says. “It’s a nice way to continue making music with others.”
Witte sees clear parallels between his musical training and his scientific work. “The skills I developed as a musician—hours-long practice, attention to small details, self-reflection, and working on my own—carry over to my PhD work,” he says.
“Doing scientific research at the bench is very similar,” he continues. “You do work with others, and that’s a really great aspect of science, but you also work alone with your own research questions. You constantly have to self-evaluate and tweak different aspects of what you’re doing so that you can make progress.”
Science and music, he adds, share a deeper resonance as well. “Music is a way of sharing something with the world that you’ve developed and created,” Witte says. “It doesn’t matter what language the audience speaks—you can all come together through music. Science can be that way as well. As scientists, we all study how life works, and we share that with others. Language helps us understand it, but at the end of the day, it’s the science that we all connect with.”
Bach’s cello suites are what I always come back to, especially after a long day in the lab. Whenever I play these pieces, it feels like home.
—Isaac Witte
Staying on Target
Now that evoCAST functions efficiently in human cells, Witte and his colleagues are focused on moving the technology toward therapeutic use. One challenge is delivery: how to get the gene-editing system to the right tissues.
“A treatment for cystic fibrosis, for instance, needs to be delivered to the lung,” Witte explains. “There are emerging technologies that are able to deliver therapeutic agents to that part of the body, but there’s still much room for progress in this area.”
Another priority is understanding evoCAST’s specificity. “We want to integrate the gene at the on-target site, but we don’t want to integrate the gene elsewhere in the genome, because that could perturb other functions of the cell,” he says. “Studying the on-target specificity of evoCAST closely is essential to ensuring that evoCAST can be safely applied as a therapeutic.”
As he prepares to graduate, Witte is energized by the work ahead—and excited to mentor new students who will carry it forward. But with cuts to federal science funding, the future is uncertain, both for potential life-saving innovations like evoCAST and for early-career researchers like Witte.
When the pressure mounts, he returns to the music that has always grounded him. “Bach’s cello suites are what I always come back to, especially after a long day in the lab,” he says. “Whenever I play these pieces, it feels like home.”
Isaac Witte's graduate training was supported by a US National Science Foundation Graduate Research Fellowship. David Liu's lab was supported by National Institutes of Health (NIH) grants R01EB031172, R01EB027793, RM1HG009490, and R35GM118062. Sam Sternberg's lab (Columbia) was supported by NIH grants DP2HG011650 and R01EB027793. Collaborators at the University of Minnesota were supported by NIH grant R01AR063070.
Banner image by George Lampe.
Get the Latest Updates
Join Our Newsletter
Subscribe to Colloquy Podcast
Simplecast





